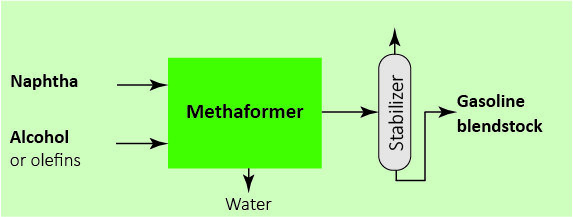
Technology
Methaforming and Aroforming technologies enable naphtha processing at small or large scale. Various types of fossil or renewable naphthas can be converted into drop-in gasoline or BTX. The processes accept heterogeneous naphthas, so that the operator can blend various types of naphtha before processing. In a large number of cases, there is no need for prior hydrotreatment.
New Alternatives to Catalytic Reforming, Isomerization, Alkylation
React naphtha with alcohols, ethers or light Olefins over proprietary catalyst. See Chemistry section below for more details.
Reduces CO₂ emissions due to reduced need for fuel gas and possibility to use renewable carbon-neutral feeds.
One-step process converts feed to standards-compliant gasoline or BTX mix.
Removes 90% feed sulfur without an external source of hydrogen, tolerant to sulfur up to 1 000 ppm.
Can process feeds that are rich in normal paraffins, e.g. Gas-to-Liquids (GTL) and renewable naphthas.
Can profitably process small streams of naphtha and other feeds, including mixed feed.
Substantially lower CapEx and OpEx relative to alternatives.
Methaforming Chemistry
The chemistry of Methaforming and Aroforming processes allows them to be tuned to produce the gasoline that meets the government specifications in one step without blending, or with minimal blending with other compounds. Key chemical reactions that form the Methaforming process are shown below. Exothermic (heat-producing) reactions are shown in red, and endothermic (heat-consuming) - in blue. The reactions don’t necessarily occur in the same order as they are shown. The choice of the specific molecule to enter a reaction (e.g. methanol, n-hexane, etc.) is illustrative of the class of molecules (oxygenates, n-paraffins, etc.).
Dehydration of alcohol, an exothermic reaction, is one of the first to take place. Light olefins that are formed in its course are intermediate products, they are used up or transformed in subsequent reactions. The water that is generated during this reaction turns to steam which prolongs the catalyst cycle by lifting the coke deposits from the catalyst as soon as they are formed.
Aromatization of olefins, an endothermic reaction, produces the components of the future gasoline that have the highest octane rating. It also releases hydrogen that may be used to hydrogenate sulfur in the feed into H2S, shifting sulfur from gasoline to LPG and fuel gas. This allows a Methaformer to process feeds with sulfur content up to 1000 ppm without prior hydrotreatment.
Alkylation of aromatics, an exothermic reaction, can take place when alcohol is dehydrated and an extremely active radical (methyl radical in this example) is formed. The radical then quickly joins an aromatic molecule (benzene in this case), forming alkylaromatics. Our proprietary catalyst promotes alkylation of undesirable benzene into valuable toluene and xylene and blocks formation of heavier molecules, e.g. fused-ring aromatics.
Aromatization of paraffins, an endothermic reaction, is another chemical pathway leading from low octane n-paraffins to valuable high octane aromatics and hydrogen. This reaction allows Methaforming to process the feeds that can not be upgraded by catalytic reforming, e.g. raffinate from the extraction of aromatics and GTL naphthas.
Isomerization of paraffins, an exothermic reaction, creates higher-octane isoparaffins from normal paraffins.